The Killers Underfoot
By Matthew Lewin
NYT/ April 14/ 2014
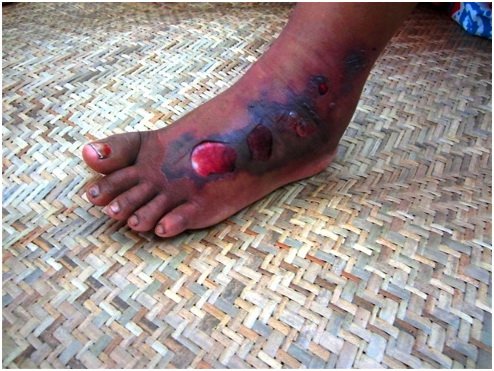 In just over a month, life-giving rains will soak India during the annual monsoons. As welcome as these rains are, they also bring with them injury and death from snakebite. As they do each year, venomous snakes seeking dry ground will slide into people’s homes, gardens and fields; they will curl up in woodpiles, latrines and play areas. Hundreds of thousands of snakebites will cause an estimated 40,000 deaths over the course of the monsoon season.
In just over a month, life-giving rains will soak India during the annual monsoons. As welcome as these rains are, they also bring with them injury and death from snakebite. As they do each year, venomous snakes seeking dry ground will slide into people’s homes, gardens and fields; they will curl up in woodpiles, latrines and play areas. Hundreds of thousands of snakebites will cause an estimated 40,000 deaths over the course of the monsoon season.
Across the globe, mainly in poor, rural parts of Africa, southern Asia and Central and South America, some five million people are bitten by snakes each year. Of those, as many as 94,000 die and another 400,000 have limbs amputated or are permanently disabled as a result. Yet public health organizations still pay surprisingly little attention to this scourge.
The World Health Organization added snakebite to its list of recognized neglected tropical conditions only in 2009, for example, and major organizations, including the Bill and Melinda Gates Foundation and Unicef, haven’t focused on the issue, which primarily affects impoverished people in agricultural regions.
Partly as a result of this neglect, progress has stalled on developing newer, effective snakebite treatments. There is no other disease or condition I know of that kills as many people in the prime of life around the globe, yet for which so few treatment innovations have been developed over the last century.
The mainstay of current snakebite treatment is antivenin — which has been produced in virtually the same painstaking way since their introduction in the 1890s by the French physician Albert Calmette. The venom is milked from the snake’s venom glands via its fangs into a cup and then injected in diluted form into a horse or sheep. The animals eventually produce antibodies, which are then extracted from the animals’ blood, freeze-dried and injected into snake-bitten humans. While these mixtures save lives when administered properly, a number of factors make antivenin less than ideal in many cases.
For one, antivenin is beyond the financial reach of most people in the developing world and expensive for most governments to subsidize. In India, the serum necessary for treatment can cost $325 or more, making it unfeasible for people earning an average of about $1 a day, according to a study of the financial impact of snakebite by a team of researchers led by Sakthivel Vaiyapuri of the Institute for Cardiovascular and Metabolic Research at the University of Reading in Britain. Add to that the cost of hospitalization and additional treatment — which can rack up costs of nearly $6,000 — and treating a snakebite can financially devastate a family for generations.
 Antivenins also vary greatly in their effectiveness. Like vaccines to cover different strains of flu, antivenins are designed to treat the most common deadly snakebites, but their effectiveness can vary depending on the diet of the snake, time of year, geographic location and quality of the antivenin manufacturing. In addition, the risk of complications from antivenin, such as anaphylactic shock, paralysis and hemorrhage, is high, which means it should be administered only in the supervised atmosphere of a hospital.
Antivenins also vary greatly in their effectiveness. Like vaccines to cover different strains of flu, antivenins are designed to treat the most common deadly snakebites, but their effectiveness can vary depending on the diet of the snake, time of year, geographic location and quality of the antivenin manufacturing. In addition, the risk of complications from antivenin, such as anaphylactic shock, paralysis and hemorrhage, is high, which means it should be administered only in the supervised atmosphere of a hospital.
Yet more than three out of four of those dying from snakebite in India never even make it to a hospital, according to the Million Death Study, a continuing study of premature mortality. While a bite itself may be painless, some venoms take effect within minutes; others take hours. Silently coursing through the body, some weaken, paralyze and then suffocate their victims. Other venoms cause the victim to bleed to death. For many of those who live in rural areas far removed from organized medical care, help comes too late.
As the expedition doctor for the California Academy of Sciences in San Francisco, I have had to grapple with the potential consequences of snakebite. Tragically, an academy scientist working in a remote Burmese jungle in 2001 lost his life to a bite from a krait, a highly venomous snake that kills by paralyzing its victims. While we equip academy researchers with almost everything they could possibly need in an emergency, no effective field antidotes for snakebite exist.
The world needs the snakebite equivalent of EpiPens: epinephrine autoinjectors used to stop allergic reactions. The ideal field treatment would have fewer side effects than antivenin and could be administered easily and inexpensively on site, before the venom has spread. Nearly every study of snakebite has shown that survival depends most on shortening the time between bite and treatment. Reducing the lag time from hours to minutes is crucial to saving lives.
Besides antivenin, the World Health Organization has long recommended a class of drugs called anticholinesterases, which can reverse paralysis, for paralytic snakebite, such as those caused by cobras, adders and possibly kraits, three major killers. In two well-designed studies conducted in the 1980s, the drugs were shown to outperform antivenin for paralytic snakebite in patients bitten by cobras. One such drug, neostigmine, is already being used in hospitals as an adjunct to antivenin, though it appears to work only for some venomous snakes. But insufficient research has been done to fully understand the potential of these drugs and, like antivenin, they can currently only be administered in the hospital setting.
Yet, in the form of a nasal spray, neostigmine could provide one safe, cost-effective solution. It is inexpensive, heat-stable, and can be absorbed quickly into the body. This class of drug has already been used experimentally in nasal-spray form for the management of diseases such as Alzheimer’s and myasthenia gravis, an autoimmune disease causing paralysis much like that of a cobra’s bite. But more testing is needed for this and antidotes to other types of snake venom.
That is why, in collaboration with researchers at the University of California, San Francisco, and under the strict supervision of anesthesiologists and an emergency medicine doctor, I tested nasal-spray neostigmine on myself. I was steadily infused with a drug to induce awake paralysis in a manner similar to cobra venom and then given the nasal spray.
Within minutes, I was back to nearly normal. Working with Stephen Samuel of the department of clinical medicine at Trinity College in Dublin, who is from a region of India dense with venomous snakes, we now have promising results with nasal spray neostigmine in animals given high-dose cobra venom. Mr. Vaiyapuri is also looking at ways to counteract enzymes in viper venoms that cause the bleeding and tissue destruction that leads to so many deaths, disfiguring limb injuries and amputations.
While ours and the efforts of others are promising, much more needs to be done. We urgently need a global health leader to take on this neglected medical issue and to foster the development of innovative treatments for snakebite. Doing so would make a profound difference in the health of millions.
Matthew Lewin is a doctor of emergency medicine and the director of the Center for Exploration and Travel Health at the California Academy of Sciences.
